Please see lesson #1 (Acidosis or Alkalosis) for the podcast on this lesson.
The acid base balance is dictated by following equation:
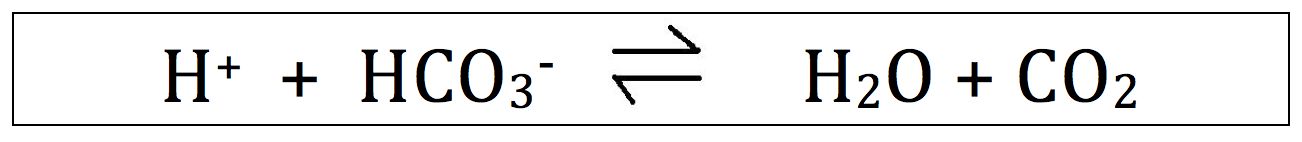
H+ is the hydrogen ion concentration. Focus your attention on him.
HCO3- is the bicarbonate, or simply "bicarb" concentration.
H2O and CO2, as you probably know, are water and carbon dioxide. Just adding this for completeness ... not to insult you.
Understanding this equation is required for understanding how HCO3- and CO2 change the pH.
Memorize this equation! This equation will make your life much easier in the acid base world.
There is a quantitative (mathematical) relationship between pH, HCO3- and CO2 and it is described by the Henderson Hasselbach equation:
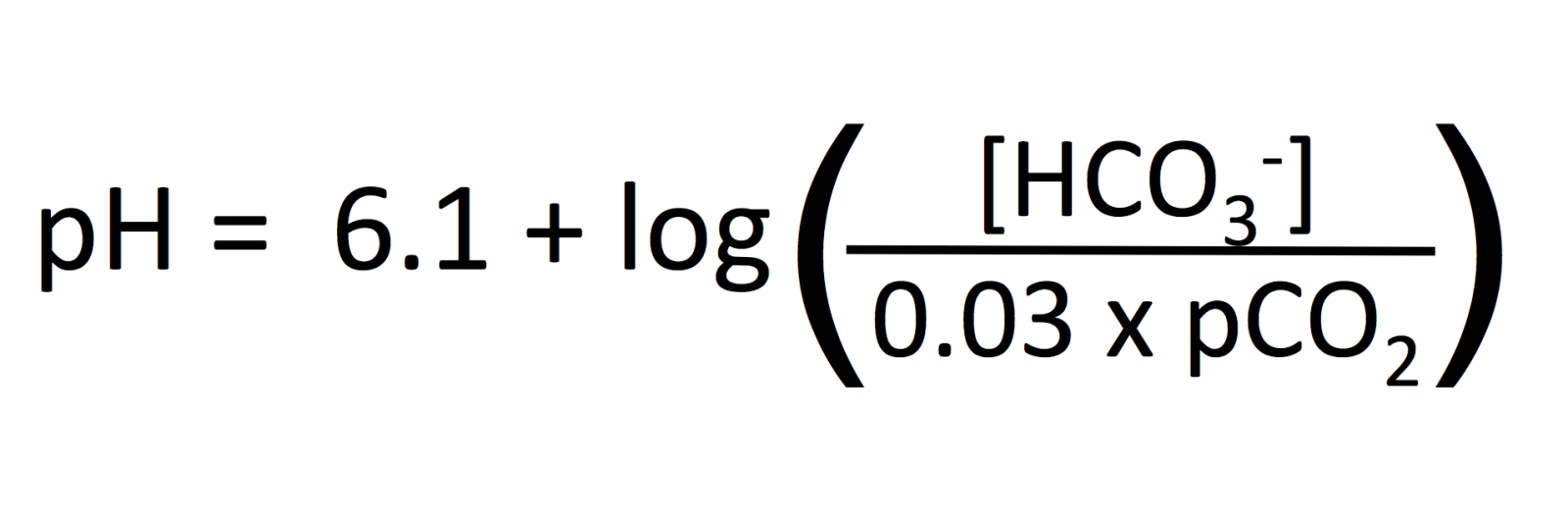
Do not memorize this equation. Simply understand that there is a fixed and well-described relationship between the 3 variables of pH, HCO3- and CO2. Given 2 of the values, we can calculate what the 3rd value is. In fact, machines that measure blood gases do this because it is easier/cheaper to measure 2 things vs. 3 things.
"Shifting" the Acid Base Equation:

- ok, let's go back to high school chemisty class
- decreasing the concentration of one chemical on the left side of the equation will shift the equation to the left
- when you shift left, all OTHER chemical(s) on the left side of the equation will increase, and all chemicals on the right side will decrease
- increasing the concentration of any chemical on the right side of the equation will also shift the equation to the left
- this means that on the right side, the other chemical(s) will decrease and all chemicals on the left side will increase
- and the opposite is true to shift the equation to the right:
- increase any chemical on the left, or decrease any chemical on the right will shift the equation to the right
- ok, there is a lot of right and left going on here and sorry if it is confusing
- bottom line: make sure you understand what happens to all chemicals in the equation if you increase or decrease one chemical. If you understand this, you are good to go!
H+ Ion Concentration Dictates the pH:- Given that pH is defined by the H+ concentration, we are primarily concerned about how changes in the other chemicals will change the H+ concentration. Memorize these 2 rules:
- a higher H+ concentration produces acidosis (low pH)
- a lower H+ concentration produces alkalosis (high pH)
- Since the "concentration of water" doesn't change, the 2 chemicals of interest are HCO3- and CO2.
- so start thinking: what will happen to the H+ concentration if we change HCO3- and CO2?
Acidosis:- decreasing the HCO3- (bicarb) concentration will shift the equation to the left and increase H+ ion concentration and generate acidosis. This process is called metabolic acidosis. The kidneys mostly control your HCO3- levels.
- increasing the CO2 will also shift the equation to the left and generate acidosis. This is called respiratory acidosis because your ventilation controls your CO2 levels.
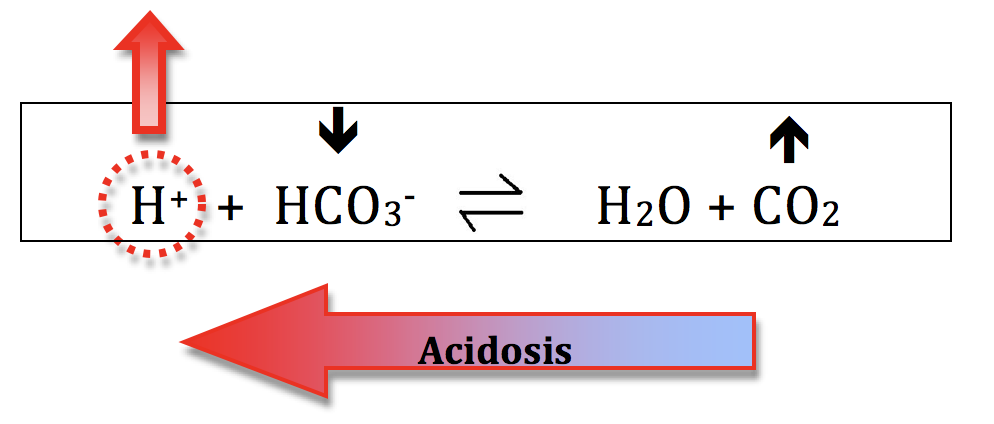
Everything discussed for acidosis can be applied in reverse, to describe processes that generate alkalosis.
Alkalosis:- increasing the bicarb concentration will shift the equation to the right and generate alkalosis. This is metabolic alkalosis.
- decreasing the CO2 will also shift the equation right and generate alkalosis. This is respiratory alkalosis.
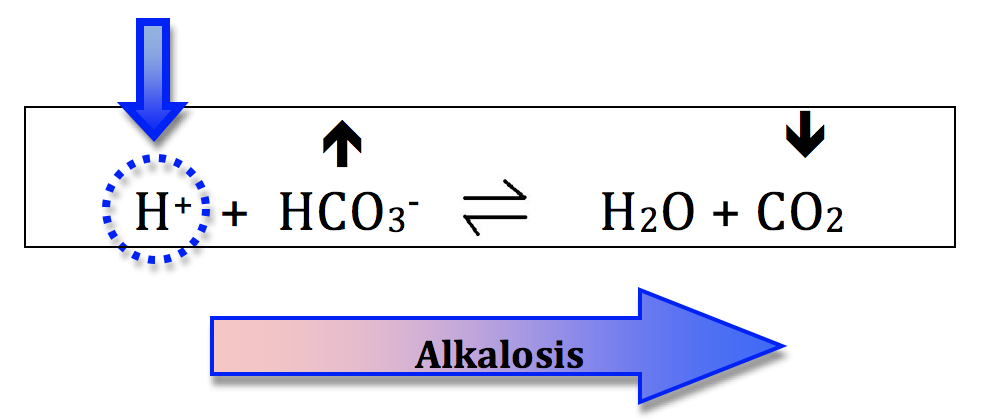
Let’s do a quick recap. There are 4 primary acid base disturbances: a) metabolic acidosis
b) respiratory acidosis
c) metabolic alkalosis
d) respiratory alkalosis
These 4 processes will form the fundamental basics of your understanding of ABG analysis. If you are confused with any of these details, it is really important to ensure you stop and re-study this lesson. If you are confused about ANYTHING on this page, you will be confused in future lessons also.